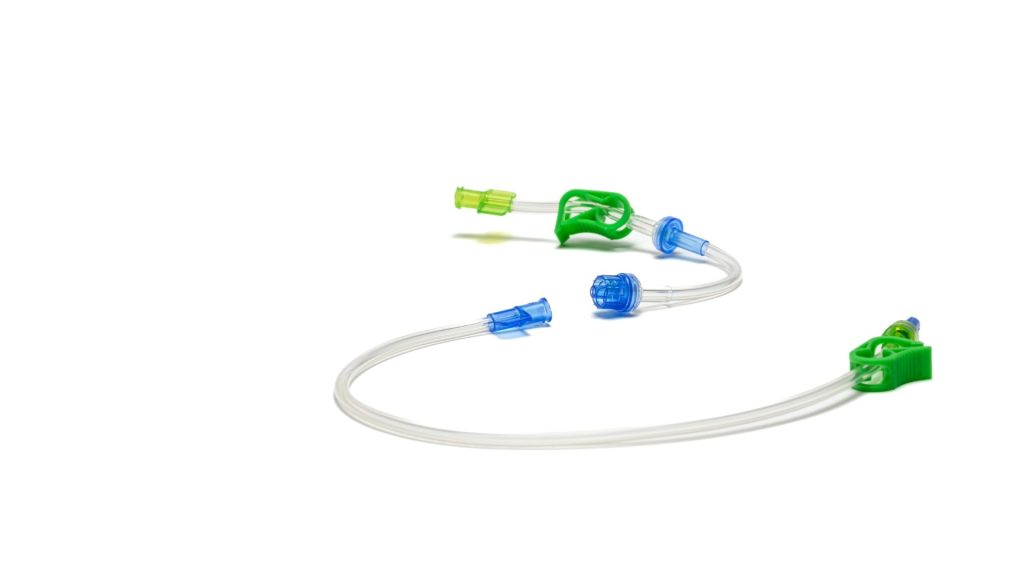
Transflux is a consumable system that allows users of syringe-based contrast injectors to leverage many of the efficiency benefits of a multi-use, pump-based system, without the need to change or modify their injector.
Naturally, with any multi-use device, patient safety and infection control are paramount. To address this critical priority, the Transflux system incorporates patented safety features, including a novel sequential dual one-way valve system housed within a designated “safety zone” on the single-use patient line.
In a new study published in the American Journal of Infection Control, researchers put this safety zone to the test. The team simulated two 12-hour imaging sessions using a popular syringe injector fitted with the Transflux consumable system. They deliberately introduced a high concentration of two challenging microbes (E. coli bacteria and MS2 bacteriophage virus) at the valves of the safety zone between each of the 51 simulated “patients”.
None of the 51 fluid samples showed any evidence of microbial cross-contamination at the Transset 24-hour day system. This robust finding confirms the system’s design, particularly the dual one-way valves, effectively prevented both bacterial and viral contamination of the 24-hour day system under laboratory conditions. By incorporating this technology along with existing infection control practices, radiology departments can confidently move toward greater efficiency and a significant reduction in plastic waste.
Vertec Scientific is the exclusive UK distributor of the popular Transflux product. Find out more and read the full peer-reviewed article here